

Zencore CHO cell line development platform can rapidly generate high yield, stable cell lines to meet your pressing timeline and ensure an efficient cell culture process development process.
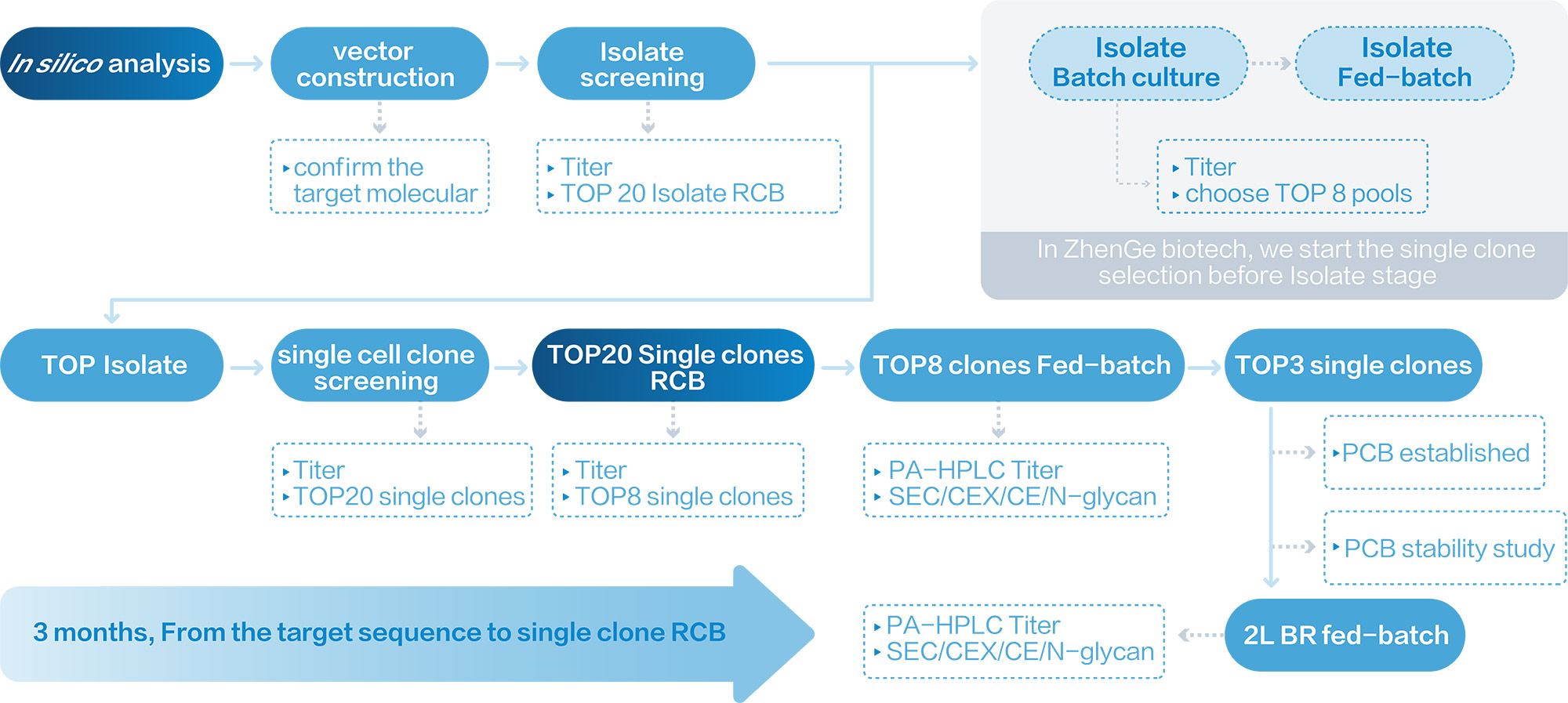
Zencore's proprietary CLD platform generates stable high yield single clones in 3 months, while traditional CLD process takes 5~6 months
Replacing traditional ELISA with ForteBio Octet has greatly improved the efficiency and accuracy for cell line screening.
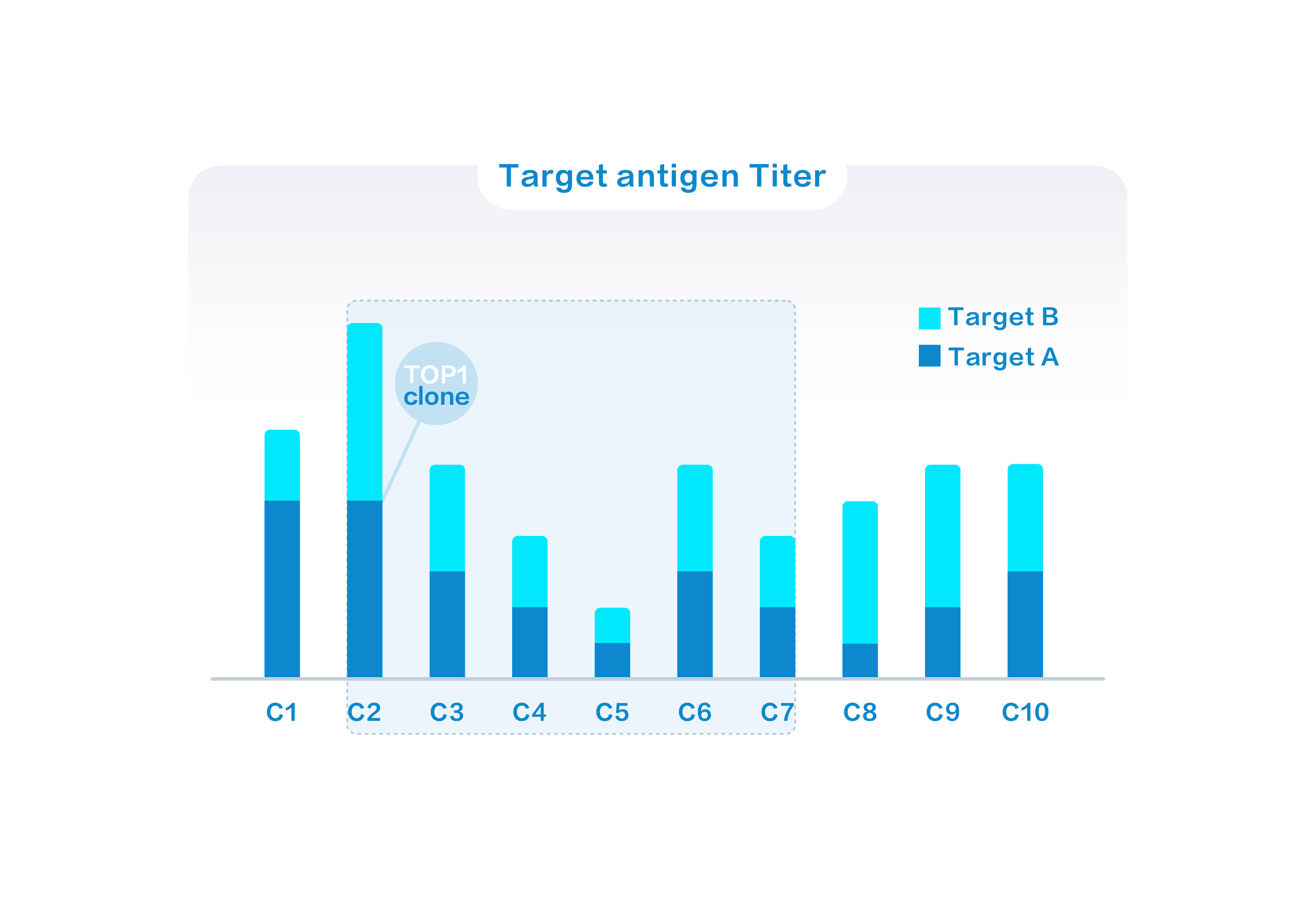
A high throughput analytical method is implemented to identify cell lines with the high titer and good quality for BsAbs CLD.
Host cell licensure for global R&D and commercialization
Cell line construction process documented compliant with global regulations
Supporting global regulatory submission
Complete host cell traceability
Industrial standard monoclonality guaranteed


Single cell printer is used for efficient single cell screening, the monoclonal selection rate is up to 90%, a 5 fold improvement over the traditional limited dilution method.
Combined with continuous imaging technology of cell culture 96-well plate, we can monitor the growth and division process of single gram cells and provide complete monoclonal traceability.
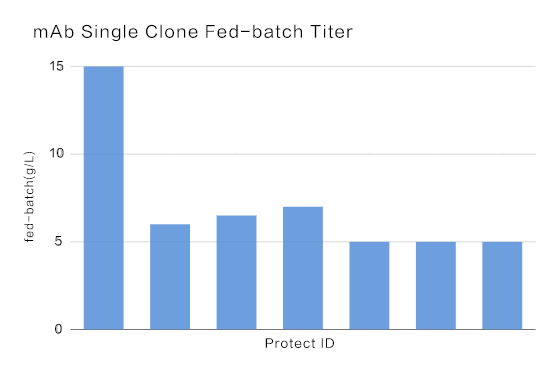
The median titer of mAb was above 5.0 g/L, and the highest titer achieved at 15.0 g/L.
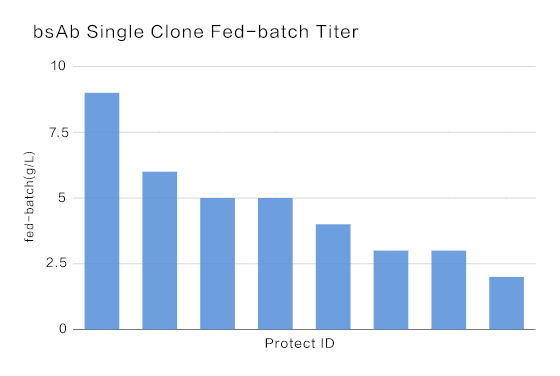
The median titer of bispecific antibody was above 5.0 g/L, and the highest titer achieved at 9.0 g/L.
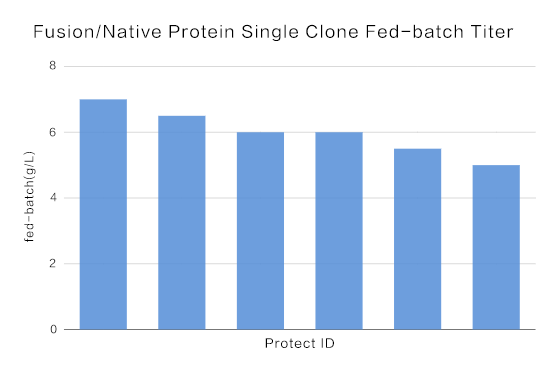
The average titer of recombinant protein was above 5.0g/L, and the highest titer achieved at 7.0 g/L.
Fully adapted ATCC CHO-K1 cells with global licensure for R&D and commercial production
Introducing ECACC CHO-K1 cells
Accepting customer-specified host cell platforms development