

Zencore Quality Management System is established based on guidelines from regulatory bodies such as NMPA, FDA, EMA, ICH and also applying situations that are applicable to Zencore GMP activities.
Zencore Quality Management System is a formalized system that documents processes, procedures, and responsibilities for achieving established quality policies and objectives. A QMS helps coordinate and direct Zenncore CDMO activities to meet client and regulatory requirements and improve its effectiveness and efficiency on a continuous basis.
The Quality Systems covers the following:
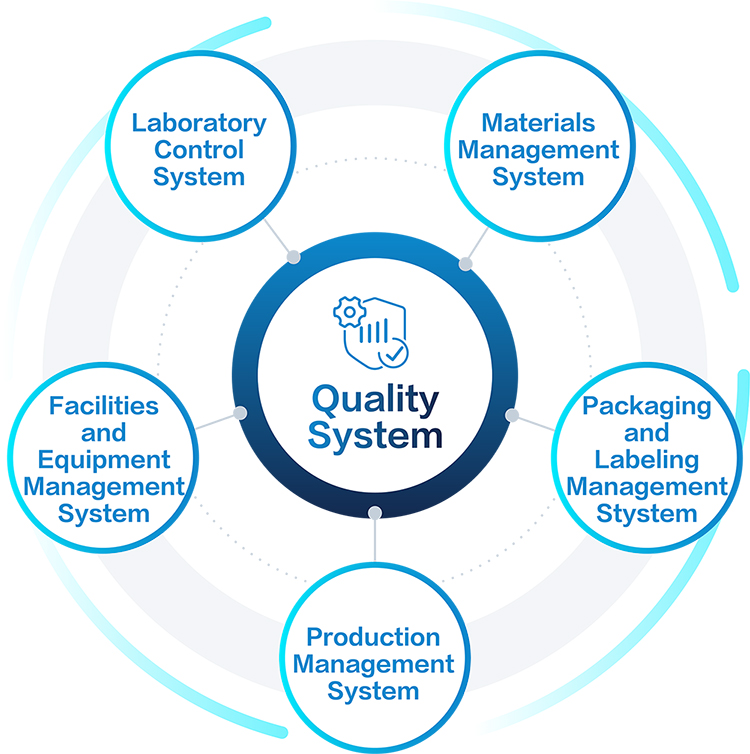
Documentation System:
Zencore Document Management System manages all GMP activities that are described in the corporate policies and standard management procedures and implemented through established standard operating procedures. Zencore Quality Assurance training department using a training plan to ensure that all personnel involved in GMP activities are qualified in the required procedures and policies. The quality assurance department has oversight the generation, review, approval, issue, archiving and other activities of all GMP documents.
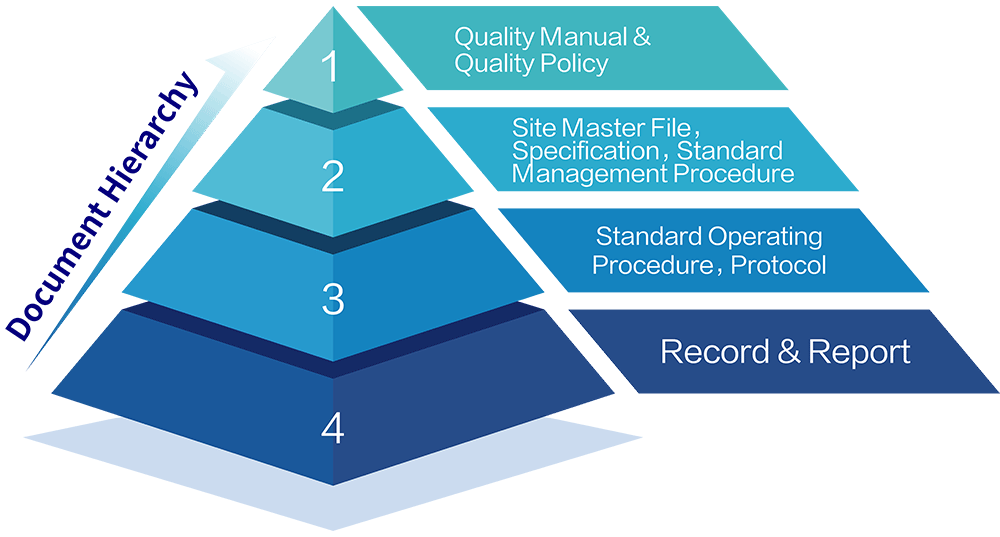
Zencore's Quality Document Hierarchy follows the standard "Document Pyramid" format, and the relationship between the document types is shown in the following figure:
Zencore Quality Management System affects every aspect of the organization's performance. It uses a systematic, disciplined approach that utilize standardized tools, established policies and procedures to manage the business and achieve customer satisfaction through continued process improvements.
The Zencore Quality System operates with the core concept of quality risk management. Zencore quality unit monitors and implements processes of all GMP activities, and timely organizes the risk assessment teams to investigate and develop appropriate CAPAs in case of deviation, OOS, complaint, return, recall and other quality events. Risk assessment shall be carried out for equipment, instruments or systems used in production and inspection, and appropriate validation and/or measurement management shall be carried out.
In order to ensure the continuous improvement of the quality system, the Quality Assurance department shall regularly organize CAPA effectiveness review, internal audit, management review, annual product quality review and other inspection or review activities, timely investigate and evaluate the quality defects found, and develop appropriate CAPA for improvement.