

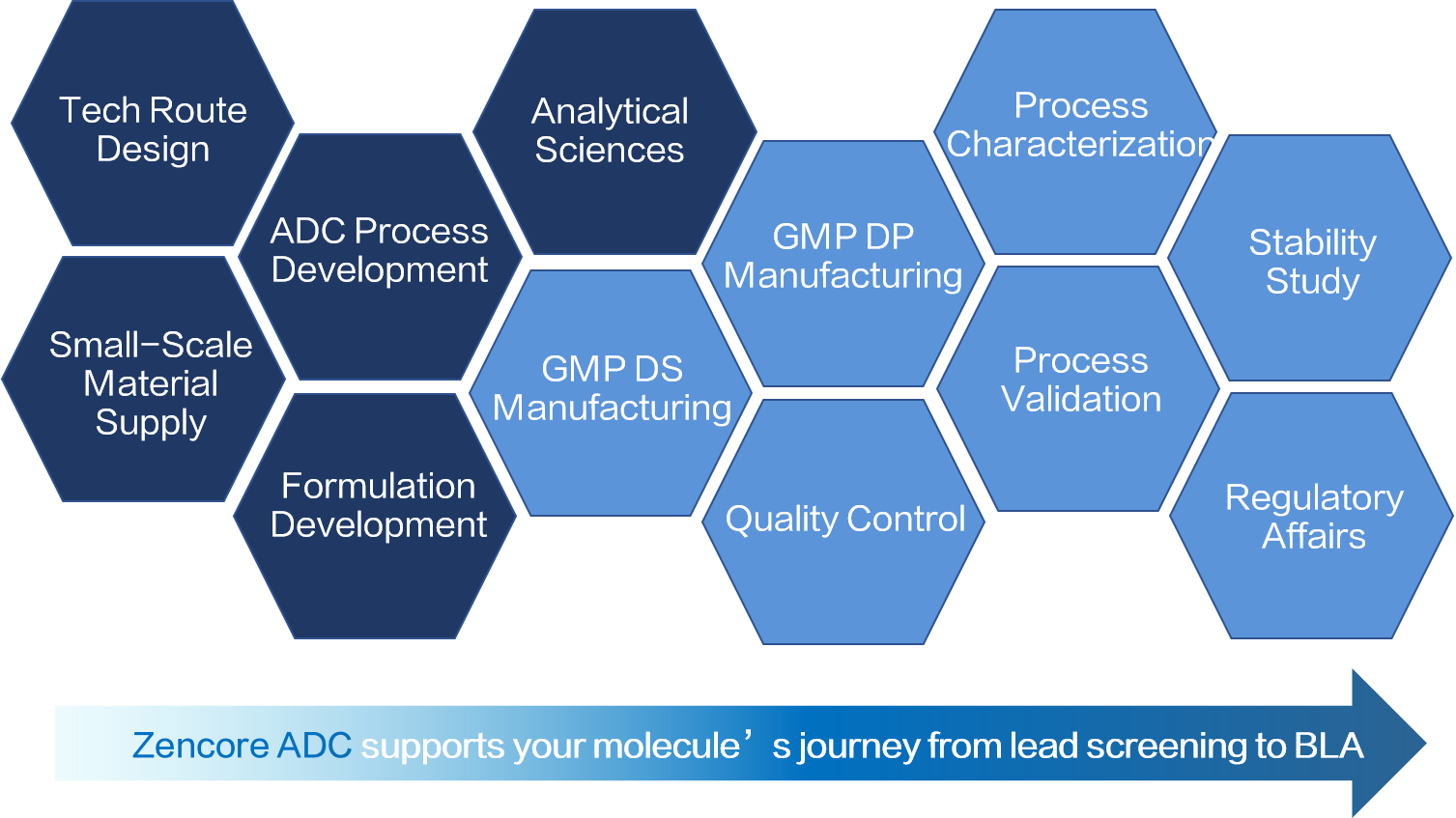
Zencore Biologics ADC R&D center was established in January 2020, when it began offerring bioconjugation drug screening and process development services. In order to further support commercial manufacturing demand, the construction of Zencore ADC manufacturing facility was initiated around the same time.
Zencore ADC R&D center offers a range of services, from proof-of-concept studies for early ADC projects to process locking for late-stage projects. As of September 2022, more than 300 ADC conjugation samples had been delivered to clients, earning their trust and praise.
Our manufacturing facility is committed to providing ADC manufacturing services for bioconjugates to both current and future clients. The Phase I construction of the facility was completed in August 2023, and it provides clients with ADC project services that meet the requirements of global IND and BLA registration, as well as commercial manufacturing. The manufacturing facility includes two drug substance (DS) manufacturing lines (for clinical and commercial production respectively), and two drug product (DP) manufacturing lines with a freeze-drying area of 5 square meters (54 sqft) and 20 square meters (215 sqft).
20400 Century Blvd, Suite 100, Germantown, MD 20874, USA
+1(240) 243-6179
Bldg 16 (C4), 356 Zhengbo Rd, Lin-gang Special Area, Shanghai, China
+86-21-6818 9888
201318
Bldg 6, 860 Xinyang Rd, Lin-gang Special Area, Shanghai, China
+86-21-6818 9888
201419
289 Zhengjia Rd, Lin-gang Special Area, Shanghai, China
+86-21-6818 9888
201419
Bldg 31 (F1), 356 Zhengbo Rd, Lin-gang Special Area, Shanghai, China
+86-21-68189888
201413
Copyright © Zencore Biologics. All rights reserved.


