Zencore Biologics provides custom media formulations tailored for client's specific CHO-based applications based on our experienced media development platform. We offer a broad portfolio of CHO media formulations for production of therapeutics in different modalities, including perfusion culture, fed-batch culture, and cell line development. The media manufactured by Zencore have been proven to be robust and high productivity. On Average, our custom-made media has shown to have a therapeutic production throughput of more than 5 g/L and as high as 15 g/L.

Highly flexible: Culture media can be tailored to any specific cell line.
Easily controlled and definite composition: Address the short-comings of commercial media.
Rapid response: We incorporate science-driven design technology into our media formulations and simplify upstream workflows.

Media formulation development
Media formulation optimization
Media pilot production in liquid or dry powder form







Cell line from client will be thawed for creating an adaptive culture and establishing a working cell line. The existing formulation and culture process will be evaluated via fed-batch (shaker flask, and bioreactor if necessary) for reproducibility.
A comprehensive quality testing process will be established and validated according to your transferred testing procedures or Zencore QC analysis platform.
The media and supplements will be determined based on their ability to support optimal process characteristics.
MVA will give support to the further formulation optimization for identifying the key nutritional components that will impact protein yield and quality.
Utilizing our platform high throughput analysis, we will be able to monitor nutrients and metabolism in CHO-based cell line. This approach enables us to optimize the regulation of cell metabolism and the reduction of by-products generated.
The correlation between key components in the media formulation and product titer will be evaluated. This enables us to further improve the output of the target protein by the optimization of the ratio of key components in the media formulation. Development, testing and manufacturing are performed at Zencore facilities, and improvement could be delivered in a shorter time than other competitors.
Once a media formulation is identified using our media platform, the cells will be grown in 2L-5L bioreactor to verify the formulation. This will enable us to further optimize cell growth, process characteristics, cellular metabolism, product yield and quality attributes.
Based on the optimized liquid formulation, the dry media powder will be developed. The process characteristics of dry powder media products will be further confirmed using a larger bioreactor (≤ 20L) to ensure the pre-defined performance criteria of dry-powder media is no less than 80% of the liquid form.
During the process development of a certain biosimilar project, the target protein exhibited oxidation and truncation issues, resulting in significant quality differences compared to the reference product. Zencore Biologics customized the media development and integrated it with upstream process and purification process to ensure that the product quality aligns with that of the reference product.
Zencore Biologics has assisted a client in enhancing the quality attributes of a biosimilar to achieve high consistency with the innovator through media screening and formulation optimization, thereby accelerating phase III clinical trials.
The Zencore media development team successfully elevated the titer of an innovative mAb to over 9.6 g/L using our custom media. Compared to mainstream commercial media, the media developed by Zencore demonstrated superior performance in cell culture.
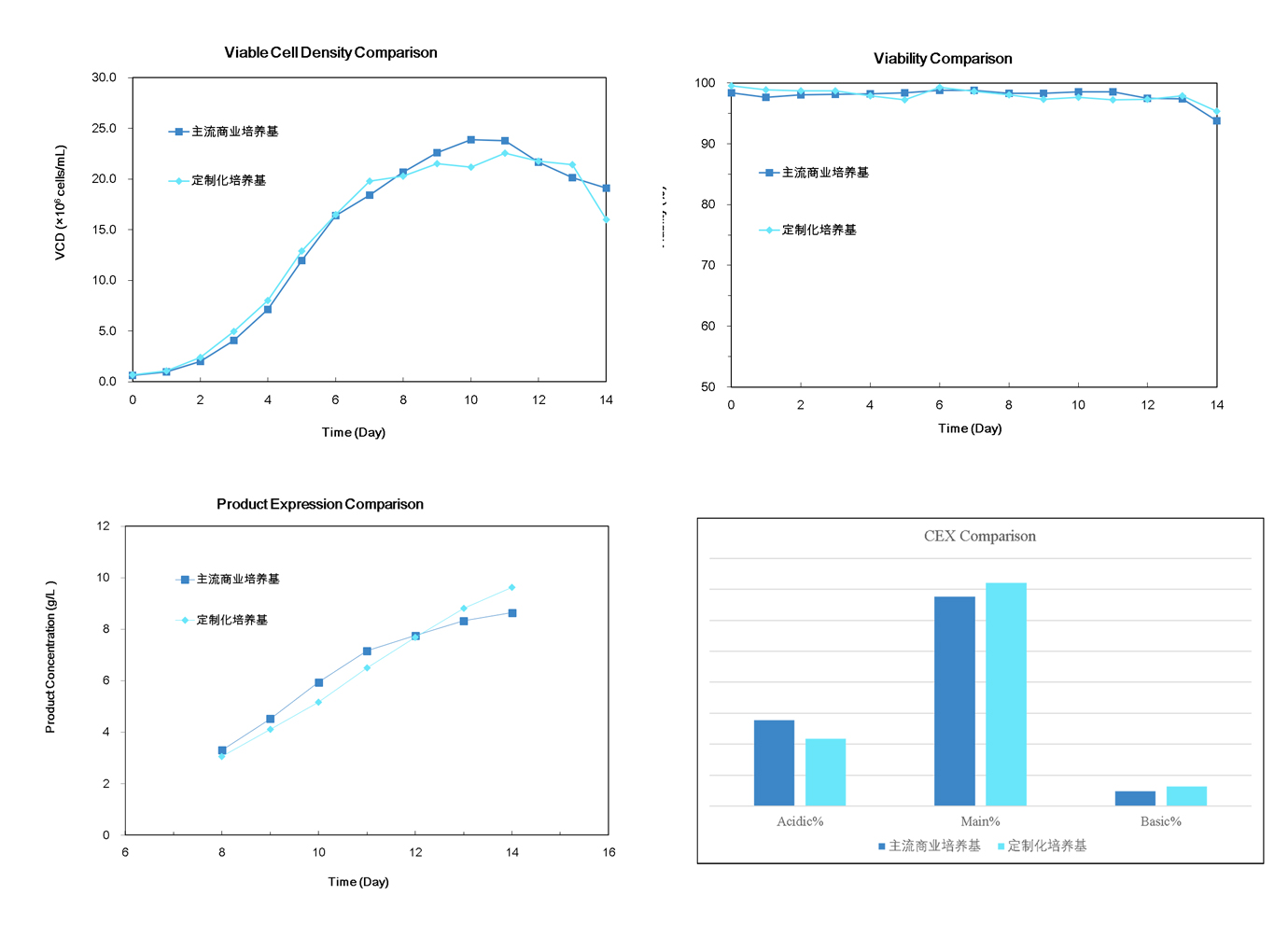
Zencore Biologics provides multiple proprietary media combinations with various nutrient formulations for CHO-based processes and can be customized for your specific needs. Using our high throughput platform, we will help you find the appropriate media formulation for selecting your primary clone and optimization, while improving the titer suitable for manufacturing. We are supporting our clients' tight timelines on a case by case basis.
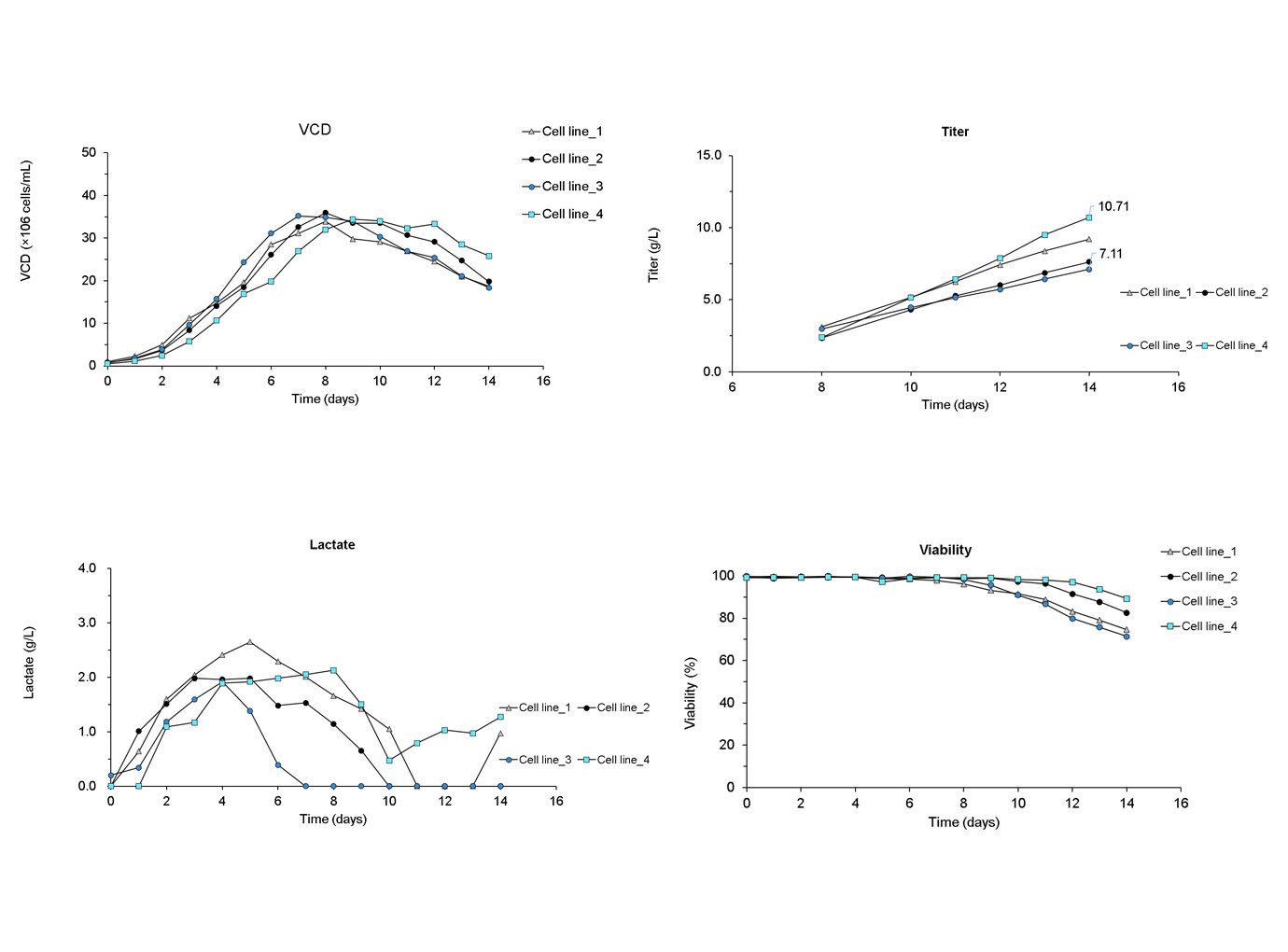
As shown below, three proprietary media by Zencore supported four different cell lines for 7.1 -10.5g/L production at the stage of clone selection and evaluation.