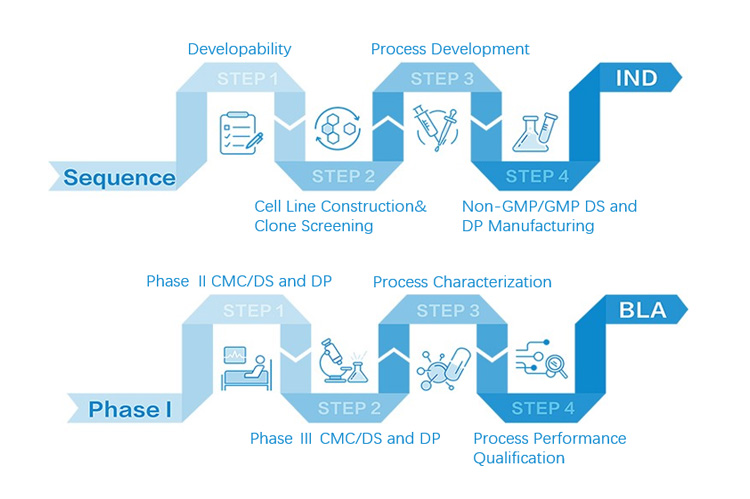
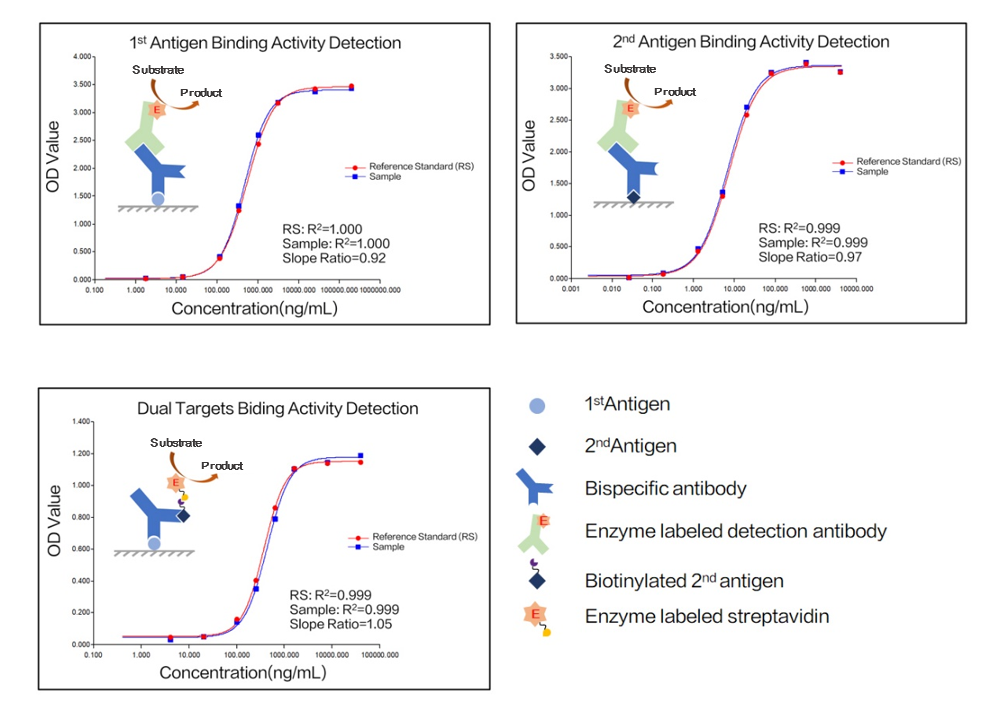
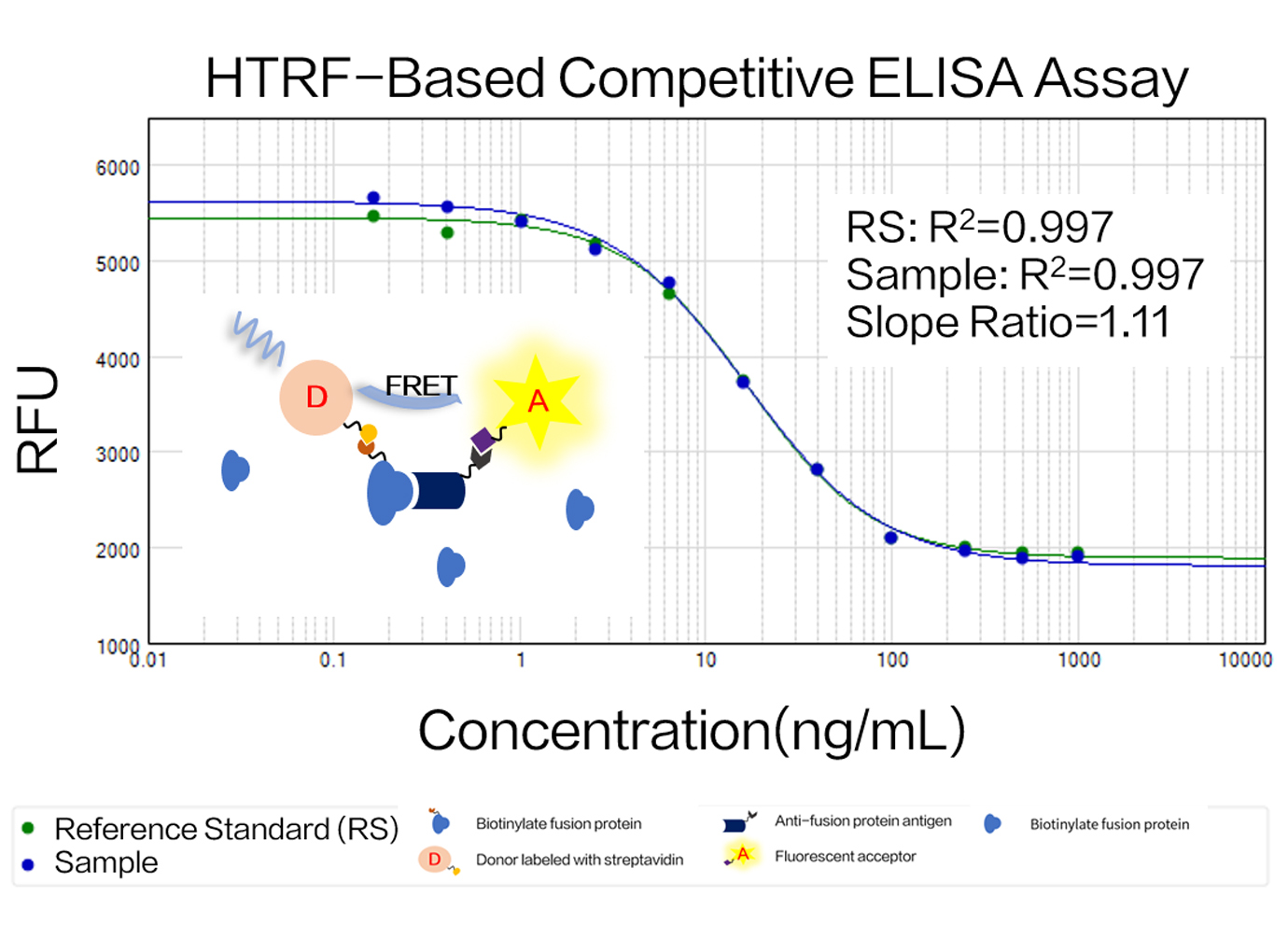
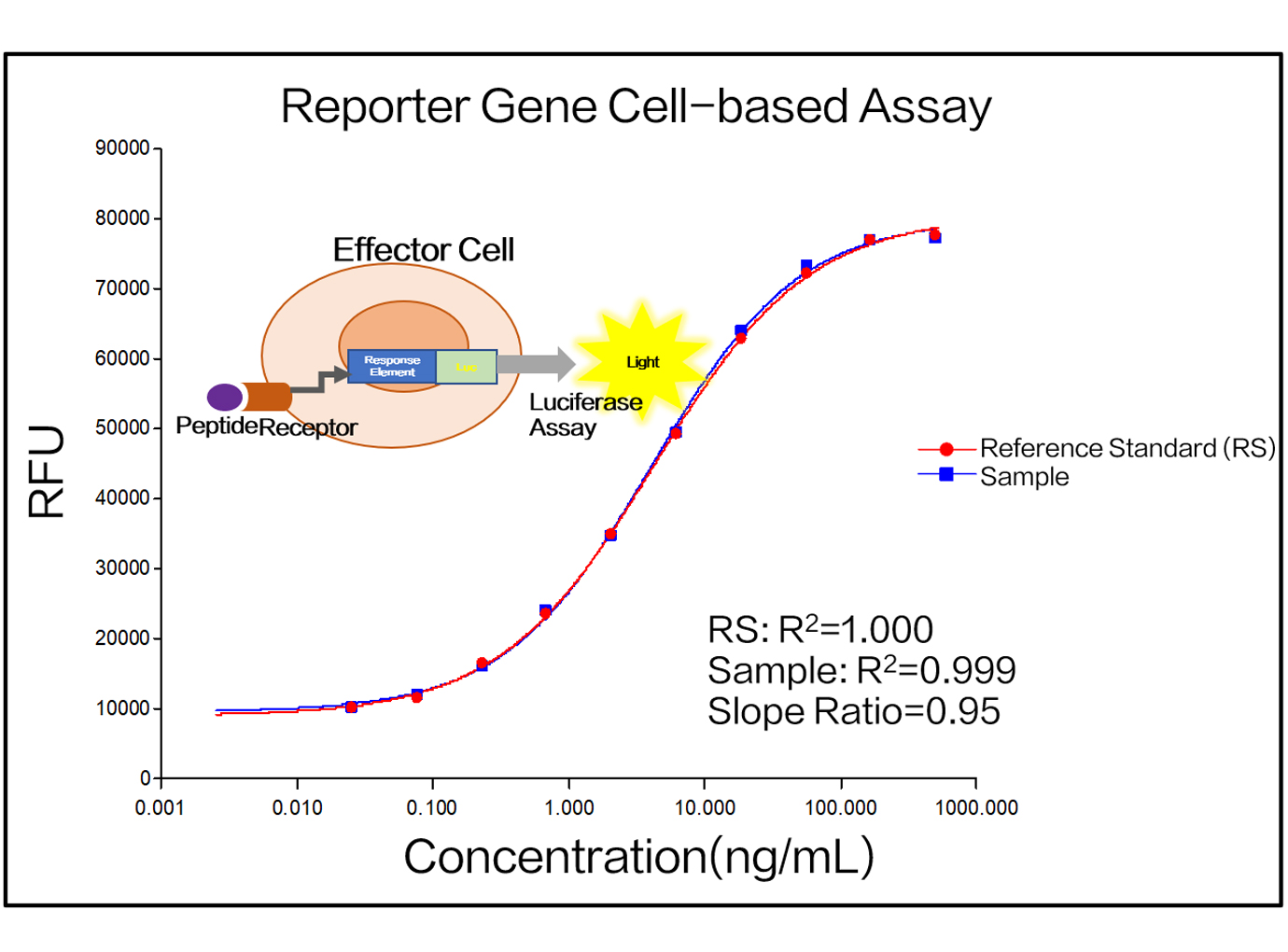
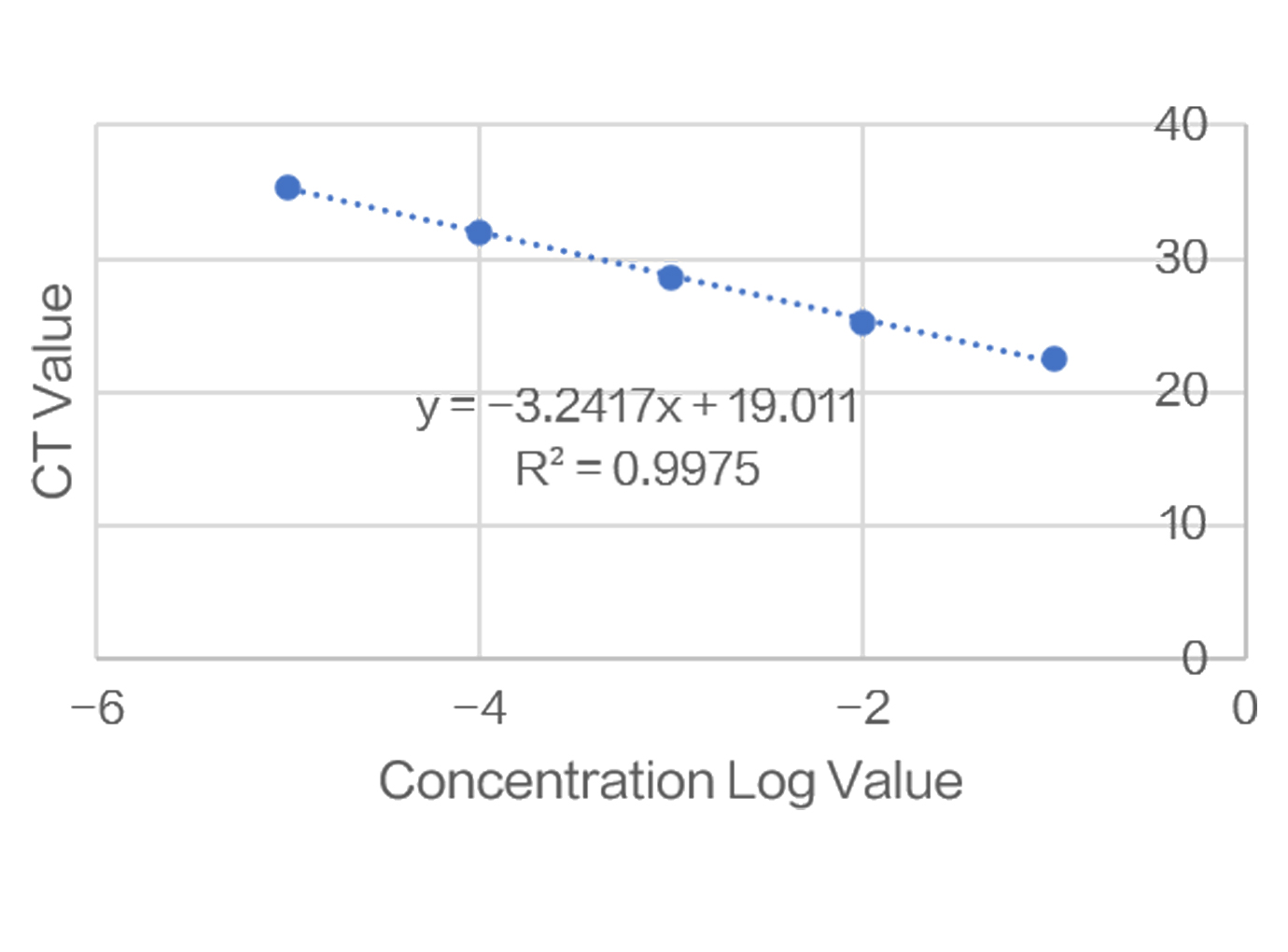
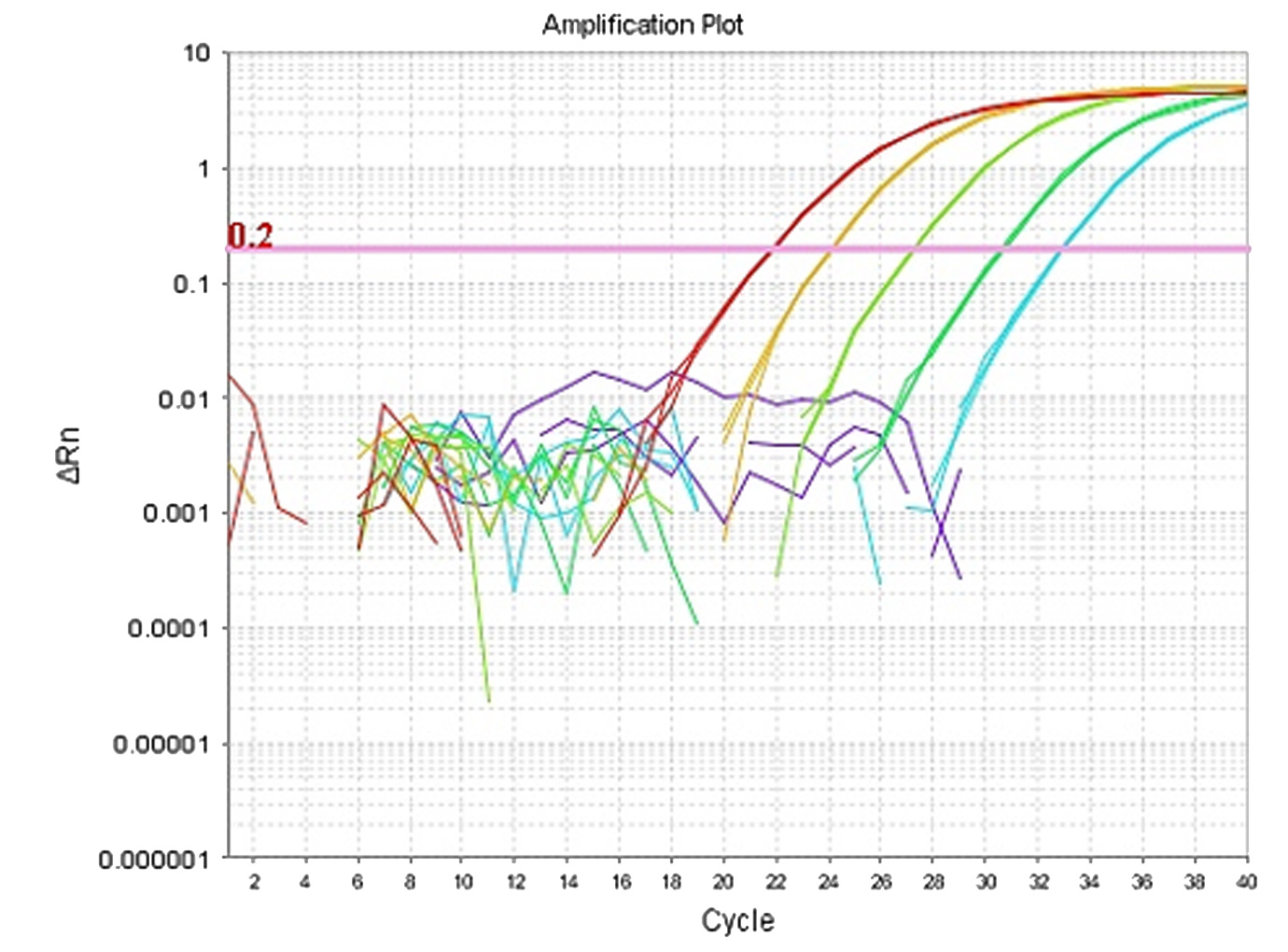
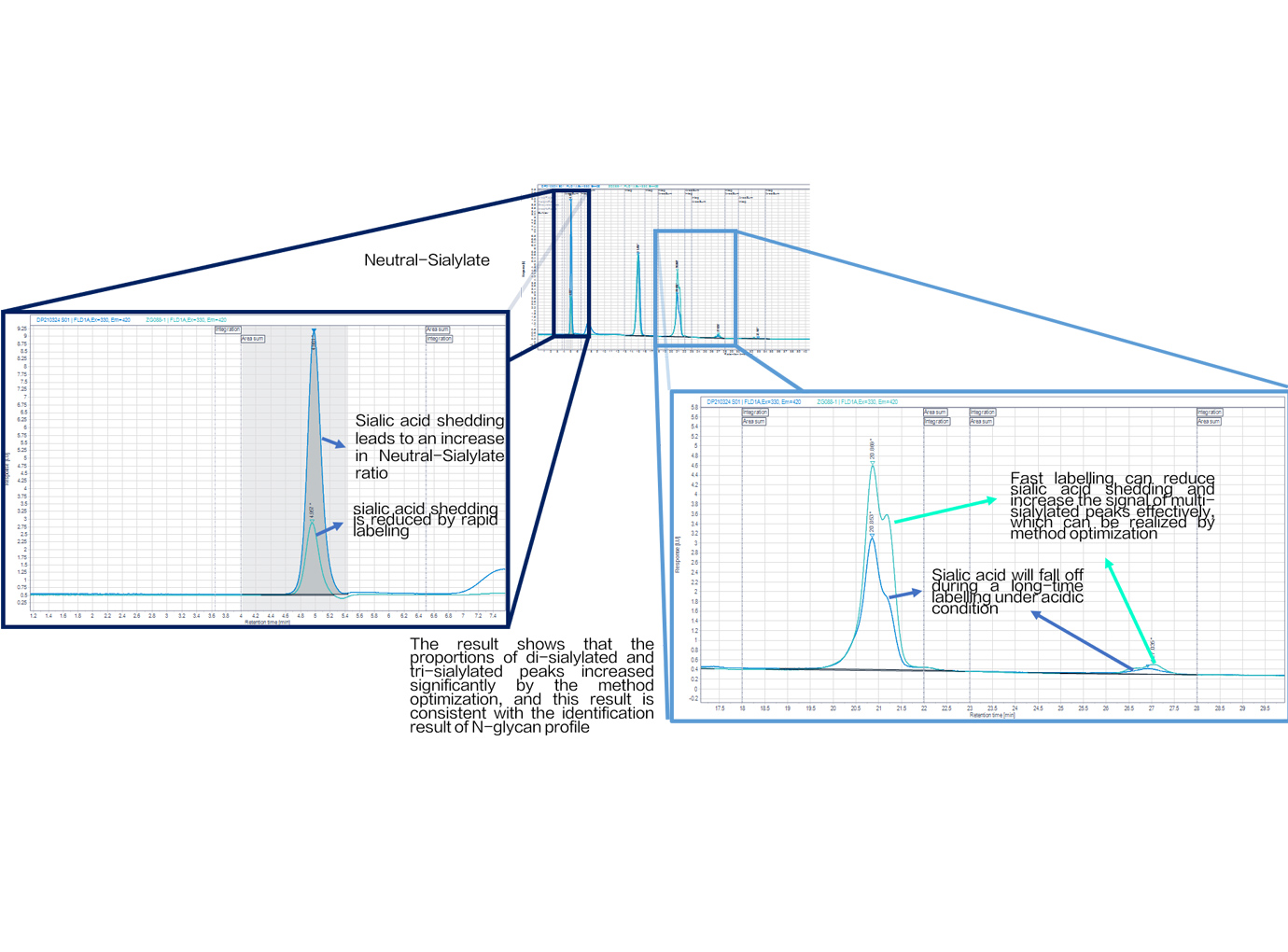
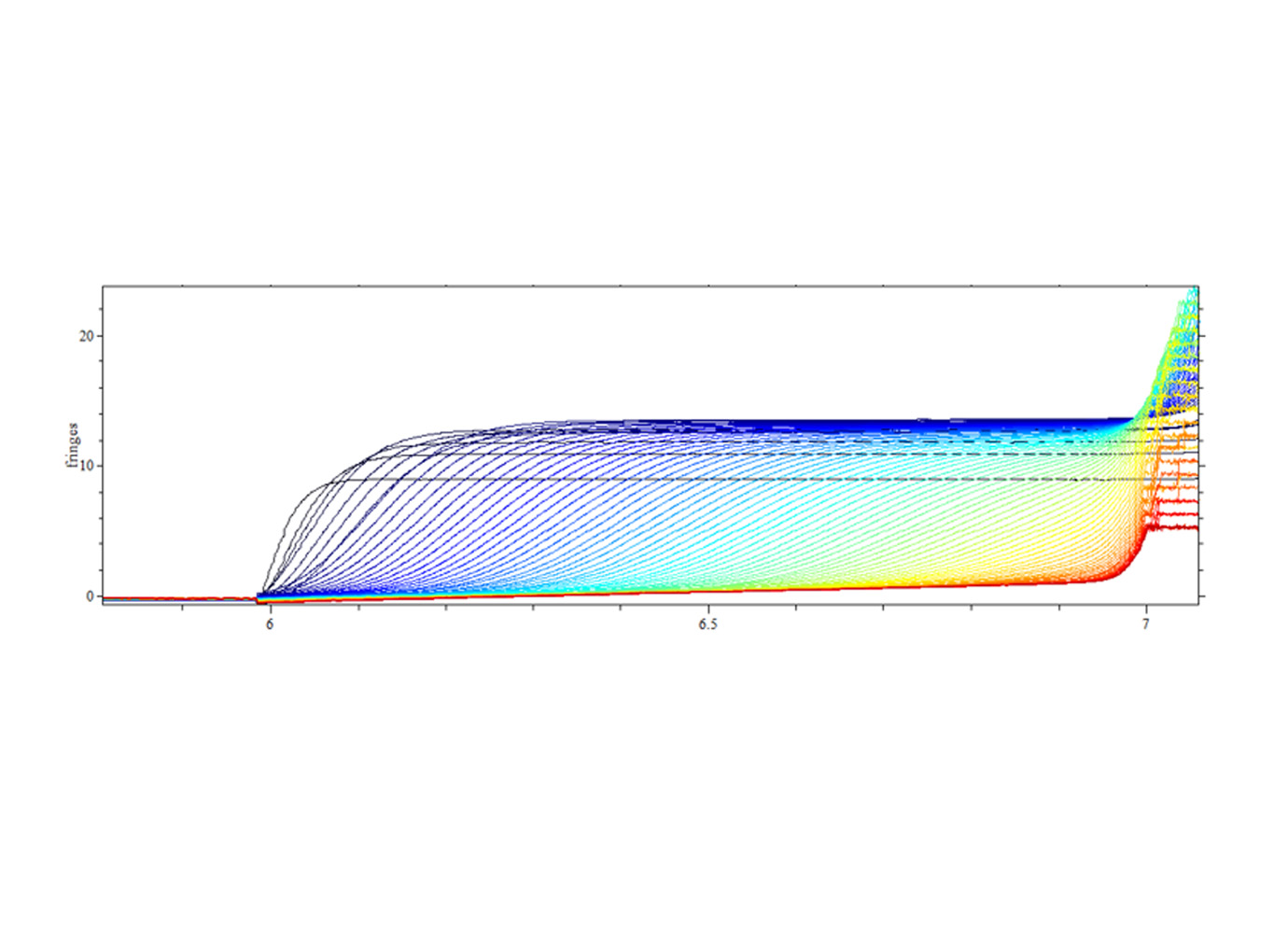
Zencore Analytical Sciences (AS) team are highly experienced in providing analytical support for biologics R&D, manufacturing, and documentation support for IND, BLA/NDA submission to FDA & China NMPA. Zencore AS team offers a comprehensive analytical strategies and solutions for biomolecules at different stages, ranging from pre-clinical to post approval stage.