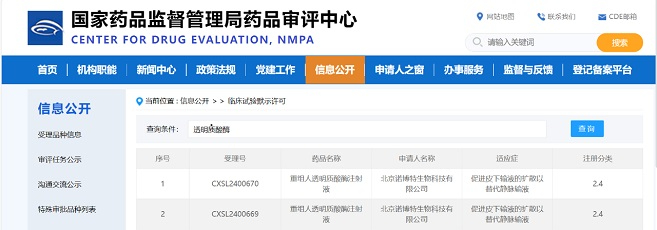
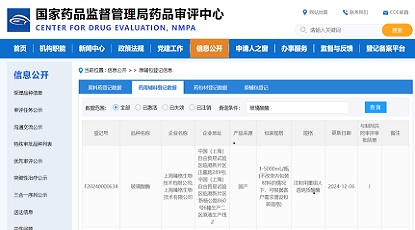
Good news for Zencore Biotech! As a biopharmaceutical company specializing in contract development and manufacturing services (CDMO), Lin-gang commercial production site of Zencore Biologics Co., Ltd. has successfully undergone a rigorous audit by an EU Qualified Person (EU QP) and obtained the Quality Person Declaration (QPD) certification. This signifies that Zencore Biotech‘s Lin-gang commercial production site has achieved compliance with GMP requirements.