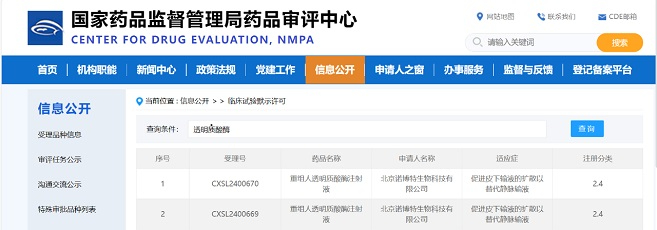
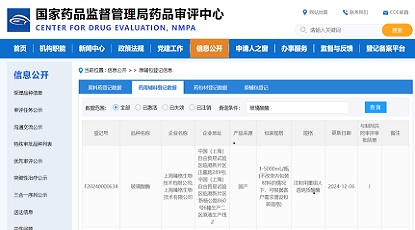
On August 5, 2023, the FDA Registration and Declaration Strategy Symposium, hosted by Zencore Biologics, successfully concluded at Zhangjiang, Shanghai. The event brought together over 50 top Chinese biopharmaceutical company founders, executives, and former FDA renowned review experts from more than 20 companies to collectively explore the current challenges in the biopharmaceutical industry.
After more than a decade of development, a group of high-quality Chinese biopharmaceutical companies have emerged, and the commercialization and internationalization of innovative drug products has become the breakthrough path for these industry leaders. To this end, Zencore's CMC, Regulatory, and Marketing departments jointly planned a invitational seminar with a global focus on late-stage product process development, commercial manufacturing, and regulatory approval, aiming to promote the collaborative development between pharmaceutical enterprises and CDMOs.

(The workshop was hosted by Dr. Wenjun Mo, Senior Vice President of Zencore, with a welcome address given by Mr. Jianxin Chen, Chairman and CEO of Zencore.)