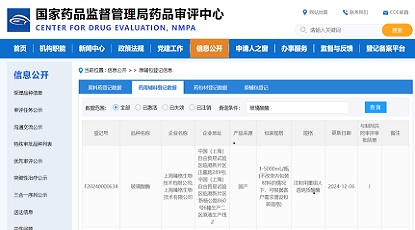
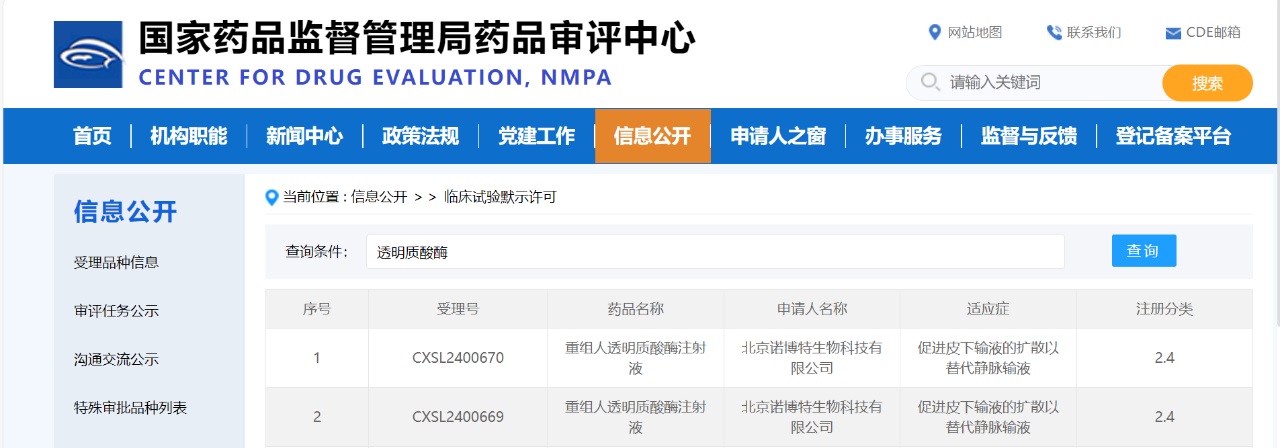
Zencore Biologics warmly congratulates Beijing Novotel Biotechnology Co., Ltd. (hereinafter referred to as "Novotel Biotech"), a wholly owned subsidiary of IMEIK Technology Development Co., Ltd., on the approval of its recombinant human hyaluronidase injection product by the National Medical Products Administration (NMPA) and the Center for Drug Evaluation (CDE) to conduct Phase I clinical trials (CXSL2400670 and CXSL2400669).
 (Screenshot from CDE official website)
(Screenshot from CDE official website)
The rHuPH20 developed by Novotel Biotech is a humanized recombinant hyaluronidase PH20 enzyme that can replace animal-derived hyaluronidase, effectively reducing the frequency of allergic and immunogenic reactions. Recombinant human hyaluronidase has a wide range of applications in fields such as assisted reproduction, antibody drug research, cosmetic surgery, gene therapy, and cancer treatment.
Zencore is honored to be the CMC provider for Novotel's hyaluronidase, offering CMC services from sequence to IND and producing recombinant human hyaluronidase with advantages including free from animal-derived contamination, high stability, and high purity. These attributes support Novotel’s new drug project in obtaining IND approval.
Zencore sincerely wishes for the clinical trial to be successful and looks forward to the product launch to market, ultimately benefiting patients. Zencore remains committed to providing global partners with high-quality, efficient, and comprehensive CDMO services.
About Recombinant Human Hyaluronidase
Hyaluronidase is widely used in medical aesthetics, ophthalmic surgery, drug delivery, and assisted reproductive technology. Its primary functions include the degradation of hyaluronic acid, promotion of drug diffusion, and prevention of postoperative tissue adhesion. The first hyaluronidase, which was animal-derived and extracted through biochemical processes, was launched in the United States in 1948 and has since been followed by several related products, such as Vitrase® and Amphadase®.
In contrast to animal-derived products, recombinant human hyaluronidase offers higher safety and addresses significant clinical demands in domestic application fields. The only globally approved recombinant human hyaluronidase, Hylenex®, received marketing approval in the United States in 2005. To date, no recombinant human hyaluronidase injection has been approved for marketing in China (Source: IMEIK Technology Development Co., Ltd.'s announcement regarding its wholly owned subsidiary obtaining the "Drug Clinical Trial Approval Notice").
About Novotel Biotech
Beijing Novotel Biotechnology Co., Ltd., which is an IMEIK wholly-owned subsidiary, was established in November 2016. The company is primarily engaged in drug development. Novotel Biotech has developed or launched a total of seven products, including recombinant human hyaluronidase injection, semaglutide injection, deoxycholic acid injection, liraglutide injection, lidocaine-tetracaine cream, and minoxidil lotion, which are at various stages ranging from Phase I clinical trials to market application submission.