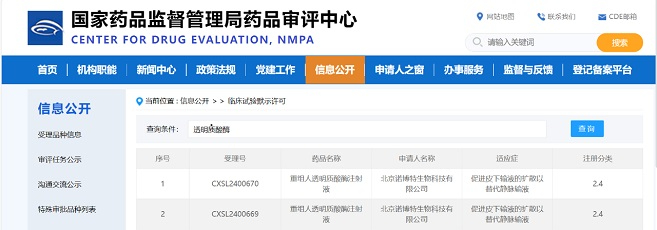
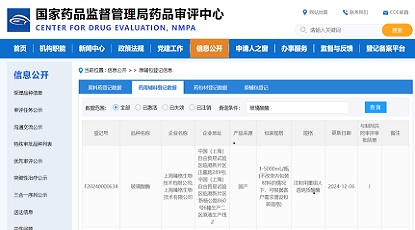
Congratulations to IASO Bio on the approval of IND application for IASO-782 by FDA and NMPA.
As the antibody CMC service provider for IASO Bio, Zencore Biologics is proud to provide Sequence-to-IND CMC services to facilitate the approval of the IASO-782 drug program in the United States and China IND.
We sincerely wish that the IASO-782 will make smooth clinical progress and be launched as soon as possible, which will bring the hope of cure to patients. Zencore Biologics will always adhere to providing high-quality, efficient and comprehensive CDMO services for global partners.