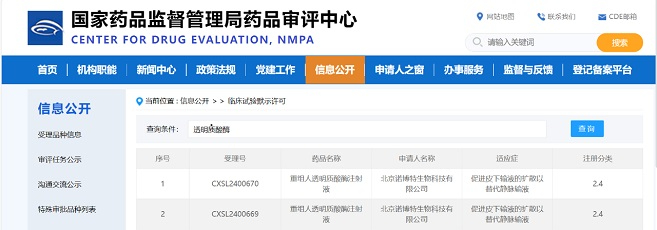
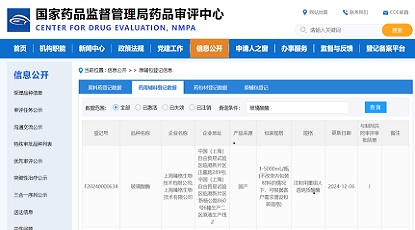
Congratulations to Xingji Biology on the approval of their IND application for XJ103 by the Center for Drug Evaluation (CDE) of NMPA. XJ103 is a recombinant humanized monoclonal antibody against Streptococcus pneumoniae, which is intended for the treatment of severe streptococcus pneumoniae infection and the prevention of high-risk groups.
As the CMC service provider of Xingji Biology, we provided CMC technology development services from DNA to IND application to help XJ103 new drug project IND approval.
We sincerely wish the XJ103 project a smooth clinical progress and an early launch to benefit patients. Zencore Biologics will continue to adhere to providing high-quality and efficient comprehensive CDMO services for global partners.