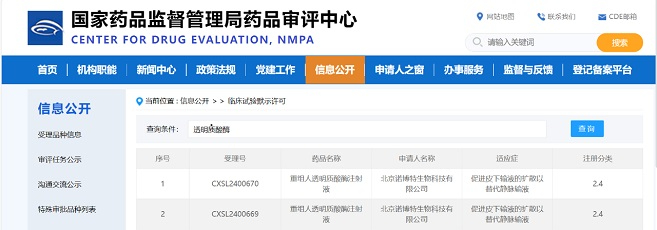
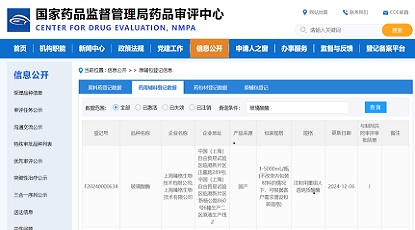
Minghui Pharmaceutical (Hangzhou) LTD. announced that the company has been granted the clearance of three IND applications from the Center for Drug Evaluation (CDE) of National Medical Products Administration (NMPA) to initiate first-in-human clinical trials in China.
Among them, MHB036C (ZG157) and MHB088C are antibody drug conjugates (ADC) generated from Minghui's proprietary topoisomerase inhibitor ADC platform, with potential utilities in a variety of solid tumors. MHB018A (ZG166) is a potential best-in-class product for treating autoimmune diseases.