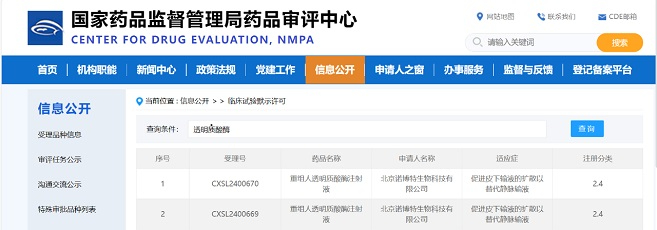
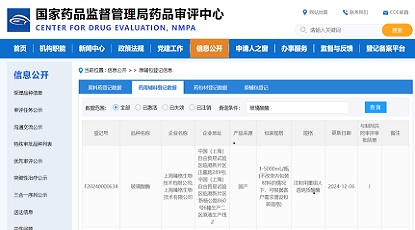
Congratulations to 4B Technologies on the successful approval of their IND application for FB1001(ZG103) by the Center for Drug Evaluation (CDE) of NMPA. We are proud to have been a part of this journey, and we are grateful for the trust that 4B Technologies has placed in us. From the initial sequencing to the final IND submission, Zencore Biologics has provided comprehensive CMC services for FB1001. Working with 4B Technologies on this project has been an incredible experience, and we are excited about the prospect of continuing our partnership in the future.