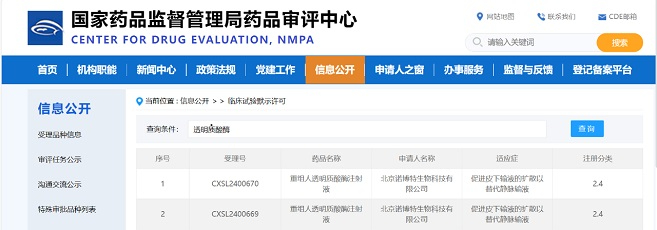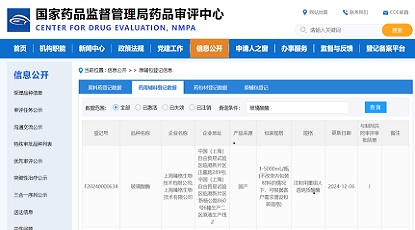
In early 2019, Beijing AbMax Biotechnology Co., Ltd. (hereinafter referred to as AbMax) successively established collaborations with JOINN Laboratories (hereinafter referred to as JOINN) and Zencore Biologics. The parties have signed collaboration agreements on providing non-clinical safety evaluation, pharmacokinetic testing research services, and complete development services for AbMax's IND submission. This collaboration will effectively help AbMax in the submission of clinical trial applications for improved anti-PD-L1 antibody drugs.
With the help of Zencore Biologics and JOINN, the preclinical research for several improved anti-PD-L1 antibody drugs based on ADCC functional technology platform and de-immunogenicity technology platform developed by AbMax will be completed soon. The parties are looking forward to more collaborations in the future.
About AbMax

AbMax Biotechnology Co., Ltd. makes use of its own intellectual property rights to protect the ADCC function technology platform and the world's only innovative technology platform to remove B-cell sites and reduce immunogenicity, to transform the already listed antibody drugs, and thoroughly solve the world problem of antibody drug resistance. It can use 1/10 of the investment and minimal risk in the shortest time, To develop effective, safe and non failure second-generation blockbuster antibody drugs.
About JOINN

JOINN Laboratories is the pioneer of commercial GLP laboratories in China, with an excellent track record spanning over two decades, and is the oldest privately-owned non-clinical safety evaluation institute. Uncompromising on quality, JOINN is the first Chinese CRO to be inspected by the US FDA for GLP compliance and to be certified by CFDA. JOINN’s facilities have been successfully inspected for GLP compliance by OECD and the Korean MFDS. We are also accredited by AAALAC International for our animal care programs. We specialize in providing preclinical and non-clinical services to pharmaceutical, medical device, chemical, biotechnology, and agricultural development companies, as well as regulatory and other government agencies. We support IND applications on clinical studies and marketing registrations.
About Zencore Biologics

Established in 2017, Zencore Biologics is a premier high-tech enterprise registered in the Lin-gang Special Area of the China (Shanghai) Pilot Free Trade Zone. Zencore Biologics has research and development (R&D) centers located in both Shanghai International Medical Zone and Maryland USA, as well as cGMP and commercial manufacturing sites in the Lin-gang Life Science Blue Bay (Shanghai).