Recently, the first analytical ultra-centrifuge (AUC) to be used for servicing the biopharmaceutical industry has been installed and validated at Zencore Biologics. Zencore Biologics officially announced the beginning of AUC technical service offering for its customers, supported by the technical expert who has served many years as the technical director of the AUC team at a well-known multi-national pharmaceutical company.
Recently, the first analytical ultra-centrifuge (AUC) to be used for servicing the biopharmaceutical industry has been installed and validated at Zencore Biologics. Zencore Biologics officially announced the beginning of AUC technical service offering for its customers, supported by the technical expert who has served many years as the technical director of the AUC team at a well-known multi-national pharmaceutical company.
Q1: What is Analytical Ultra-Centrifugation?
A: Analytical ultracentrifugation is a technology to study the hydrodynamic characteristics of solute molecules by analyzing the motion trajectory of solute (such as protein) in liquid environment under centrifugal force. The instrument used in this technology is called analytical ultra-centrifuge (AUC).
In essence, AUC is an ultracentrifuge with optical detection system and has the function of detecting the movement of solute during the centrifugation process. The working principle is: under the centrifugal force, the solute in the sample solution moves along the direction of centrifugal force, which results in the generation of solvent / solute interface in the sample solution; When centrifugal is carried out at a higher and higher speed, the interface will move towards the bottom of the sample chamber along the direction of centrifugal force until it disappears; The shape and velocity of the interface are influenced by the molecular weight of the solute, the shape of the solute molecule, whether the solute molecule has self-polymerization, the density of solvent and viscosity.
The optical system of AUC can record the interface changes (changes of light absorption or interference light). By analyzing data recorded by the optical system, the molecular weight, sedimentation coefficient, monomer, polymer and fragment content of any protein in the sample can be determined. This type of ultracentrifugation is called velocity deposition. When the centrifugal speed is small, the concentration of solute increases along the moving interface; When the concentration reaches a certain value, the solute movement (the same as the centrifugal force direction) caused by centrifugal force is balanced out by the solute movement caused by diffusion (opposite to the direction of centrifugal force). Therefore, the shape and position of the interface will not change again. The shape and position of the "stationary" interface are determined by the molecular weight and concentration of solute, whether solute molecules have reversible self-polymerization. Therefore, by analyzing the morphology of the interface, the molecular weight and diffusion coefficient of the solute can be measured. The technique can also be utilized to study the reversible self-polymerization characteristic of solute. This analytical ultracentrifugation technology is called equilibrium separation.
Q2: How can AUC be used in Biomacromolecules?
A: In the biopharmaceutical industry, AUC technology has the following applications:
Determine the protein molecular weight and sedimentation coefficient;
Measure the protein monomer, polymer and fragment content;
Evaluate the reliability of size exclusion chromatography (SEC), also call gel filtration chromatography, support the establishment of SEC method;
Study the reversible self-polymerization of drug protein in different formulations; evaluate the effect of reversible self-polymerization of protein on the stability of drug protein and the immunogenicity risk of drugs;
Study the interactions between protein and protein, as well as between protein and small molecule.
Q3: What are the advantages of AUC analysis?
A: Compared with SDS-PAGE (sodium dodecyl sulfate polyacrylamide gel electrophoresis) and mass spectrometry, AUC analysis can be carried out in the native conformation of the drug protein, avoiding the damage to the protein, without the need for labeling and reference standard.
The AUC simplified the detection process. Samples can be recovered. Compared to SEC method, AUC analysis is carried out in solution without the need of supporting stationary phase, which avoids the estimation error for protein molecular weight due to the interaction between protein and supporting stationary phase. Furthermore, it can also avoid the underestimation or overestimation of polymer or protein fragment content caused by this interaction. Therefore, in recent years, FDA requires that the SEC test results have to be supported by AUC data.
Q4:What are the related analytical services that ZhenGe Biotech can provide?
A: The AUC at Zencore Biologics has both UV and interference detectors. The temperature control system can stably control the temperature for analysis process between 4 ℃ and 40 ℃. The system can process a maximum of 7 samples at the same time. Therefore, it can provide with the customers the following technical services or more:
Measurement of protein monomers, polymers and fragments content for drug substance or drug product;
Determination of the protein molecular weight in native conformation;
Support the SEC method by comparing the results of AUC with SEC;
Determination of the reversible self-polymerization of drug protein in different buffers. Characterization of the reversible self-polymerization to support the development of formulation buffer. We will evaluate whether reversible self-polymerization affects protein stability and the risk of immunogenicity.
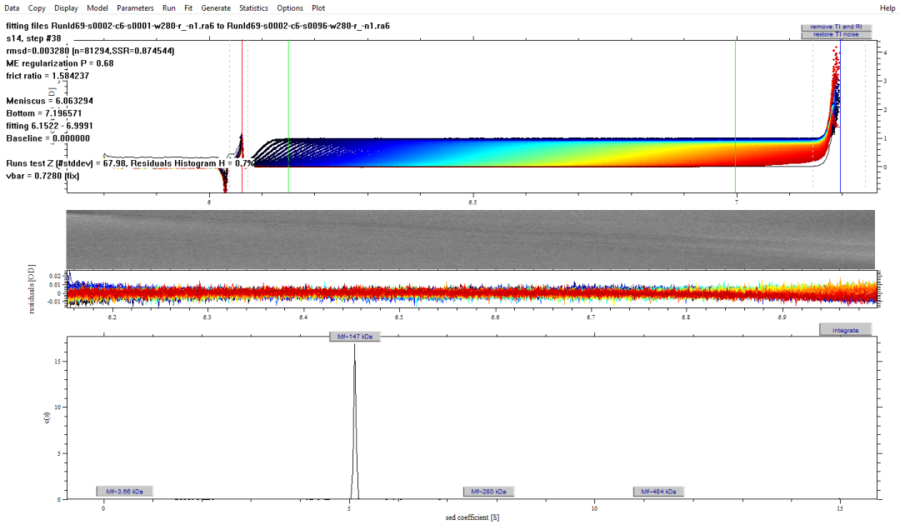
The above figure shows the UV scanning data of the sedimentation rate of a monoclonal antibody sample from a customer (the figure shows the distribution after sedimentation at 40,000 rpm, wavelength at 280 nm, and the sample concentration is 0.5 mg / ml).
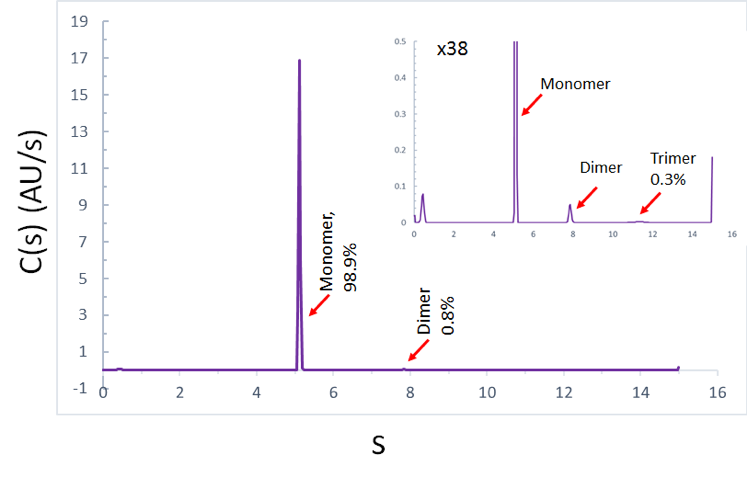
The above figure shows the sedimentation rate, molecular weight distribution and ratio of a monoclonal antibody monomer, dimer and trimer, respectively.