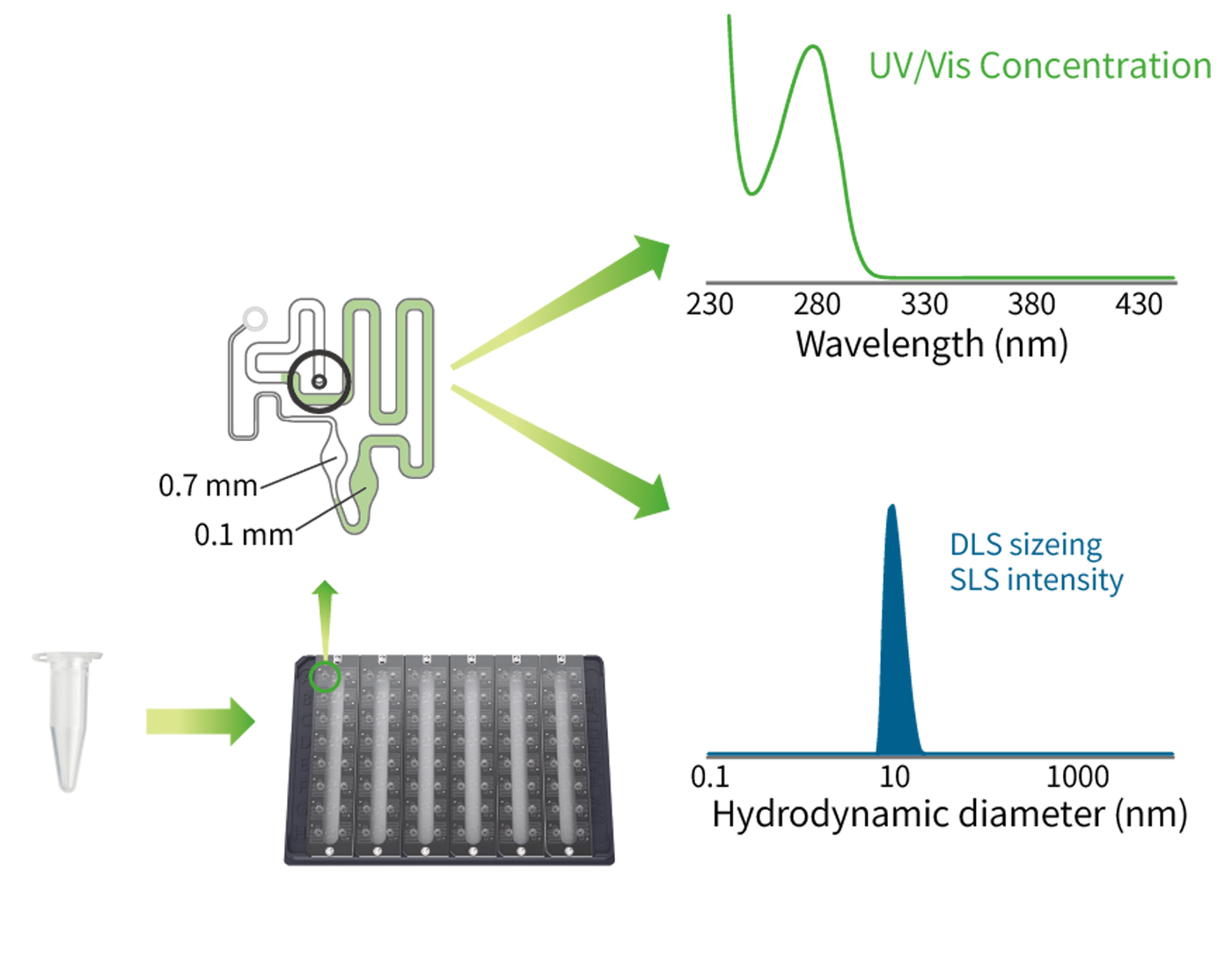
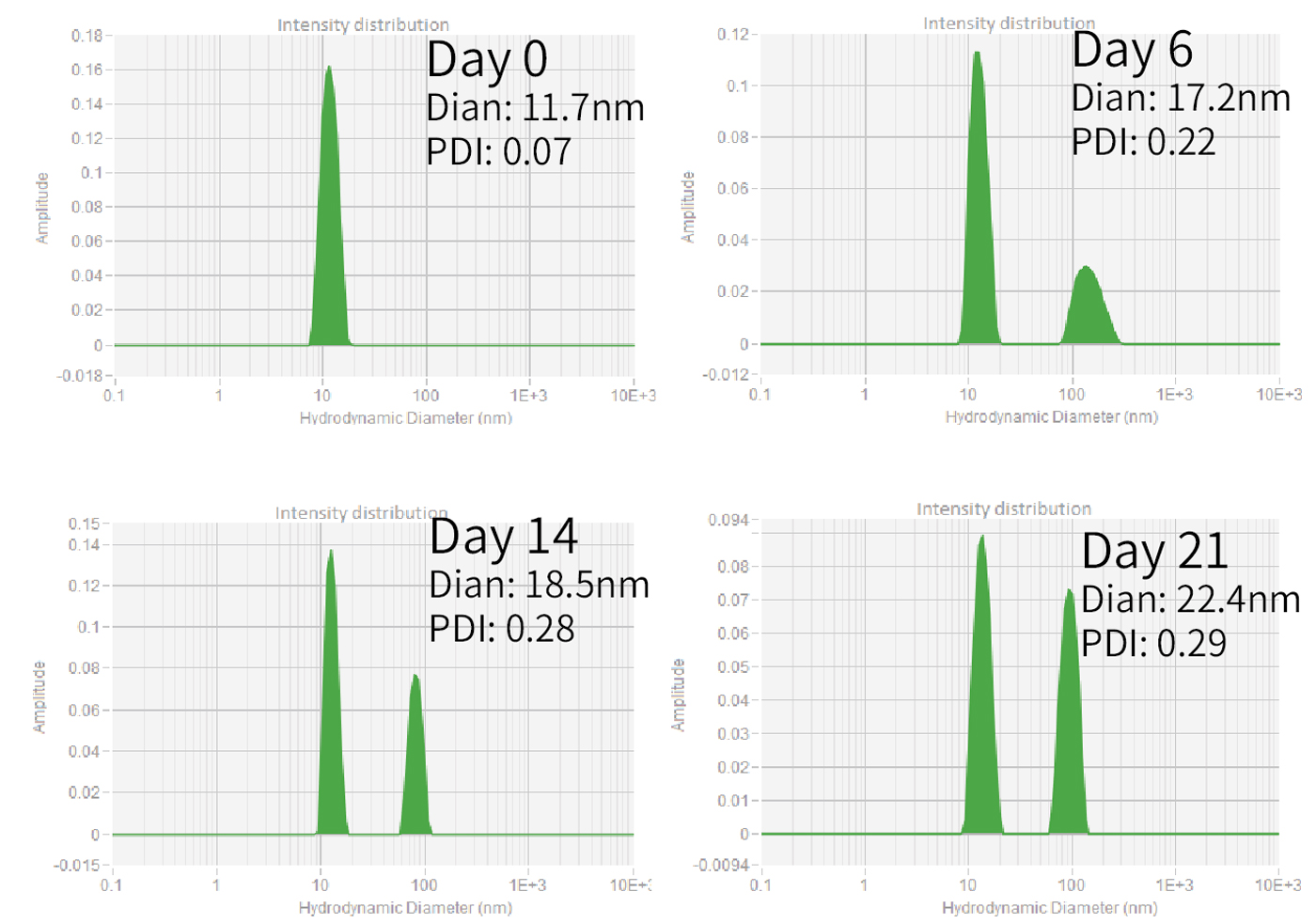
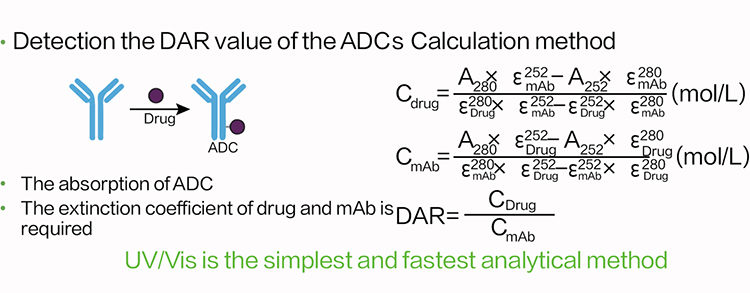
Zencore offers AUC technical services to our clients, which was the first to provide AUC services to biopharmaceutical industry in China. The AUC team at Zencore is led by a chief scientist who used to be the head of AUC technology in a multinational corporation for many years. AUC can be used to characterize purity, molecular weight, shape, and interactions of biological molecules in their native state.
Q1: What is AUC?
A: AUC is a technique for studying the hydrodynamic characteristics of soluble molecules (such as protein) by analyzing their motion trajectories in solution under the action of centrifugal force. Two kinds of experiments, time-dependent and time-independent, can be achieved, respectively named sedimentation velocity (SV) and sedimentation equilibrium (SE).
In SV experiments, an initially uniform sample solution will produce a solvent-solute interface as the solute is trailed by centrifugal force. The interface will move along the direction of centrifugal force until it reaches the bottom of sample cell. The boundary and moving rate of the interface are affected by the molecular weight, molecular conformation and self-aggregation property of the solute, the density and viscosity of the solvent, and other factors. The optical system of AUC can record the change of interface by detecting light absorbance or interference. By analyzing the data, molecular weight, sedimentation coefficient, purity of protein monomer, polymer and fragment can be determined.

In SE experiments, sample solution is centrifuged at a lower angular velocity than is required for an SV experiment. As the solute concentration near the bottom gradually increases, two opposing processes of sedimentation and diffusion will reach an equilibrium ultimately, therefore the resulting solute distribution is invariant with time. The shape and position of the "fixed" interface is affected by the molecular weight, concentration, and the reversible self-aggregation of solute, etc. By analyzing the morphology of this interface, solute molecular weight, diffusion coefficient and other parameters can be measured, and the characteristics of reversible self-aggregation can be studied.
Q2: How to apply AUC in the analysis of biological macromolecules?
A: In the biopharmaceutical industry, AUC technology has the following applications:
1. Determination of molecular weight, sedimentation coefficient and diffusion coefficient.
2. Purity determination of monomers, polymers, and fragments.
3. Orthogonal method to evaluate and support the establishment and confirmation of size exclusion chromatography (SEC) method.
4. Powerful tool to study the reversible self-aggregation property, stability, and immunogenicity of proteins in different formulations.
5. Strong method to study the interaction of protein-protein, DNA-protein, small molecule-protein, ligand-acceptor and so on.
Q3: What are the advantages of AUC compared with other relevant protein analysis methods?
A: Compared with sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and mass spectrometry, proteins can be characterized in the native state by AUC, avoiding the interference and damage from working buffer, labeling group, internal standard and any other reagents. It makes the detection process more convenient, and sample can be recovered. Compared with SEC, proteins are separated in a single aqueous phase, avoiding the interaction between protein and stationary phase. It eliminates the deviation in estimating molecular weight and protein content of polymer or fragments caused by this interaction. Therefore, FDA suggested that AUC could be an orthogonal method to support and confirm the SEC results in the BLA declaration stage.
Q4: What kind of analytical services can be provided by Zencore?
A: AUC in Zencore has both absorbance spectrometer and Rayleigh interferometer, also equips with a temperature control system of 4℃-40℃. Seven samples can be analyzed at the same time. It can help customers with the following technical services:
1. Analysis of the content of monomers, polymers and protein fragments in drug substances or drug products.
2. Determination of molecular weight of protein in natural state.
3. Support the SEC method development by comparing the results from AUC and SEC.
4. Determine the reversible self-polymerization of drug proteins in different buffers and analyze the characteristics of the reversible self-polymerization detected to provide a basis for the development of formulation buffer.