Congratulations to D2M Biotherapeutics (Beijing) Ltd. (“D2M Biotherapeutics”) on the approval to conduct Phase I clinical trial (CXSL2400035) in China by the China National Medical Products Administration (NMPA) China Drug Evaluation Center (CDE) for their first pipeline R&D product, DM919, a pan-tumor type adaptive checkpoint inhibitor monoclonal antibody. The pipeline was officially approved by Food and Drug Administration (FDA) on December 21st, 2023 (NCT06328673).
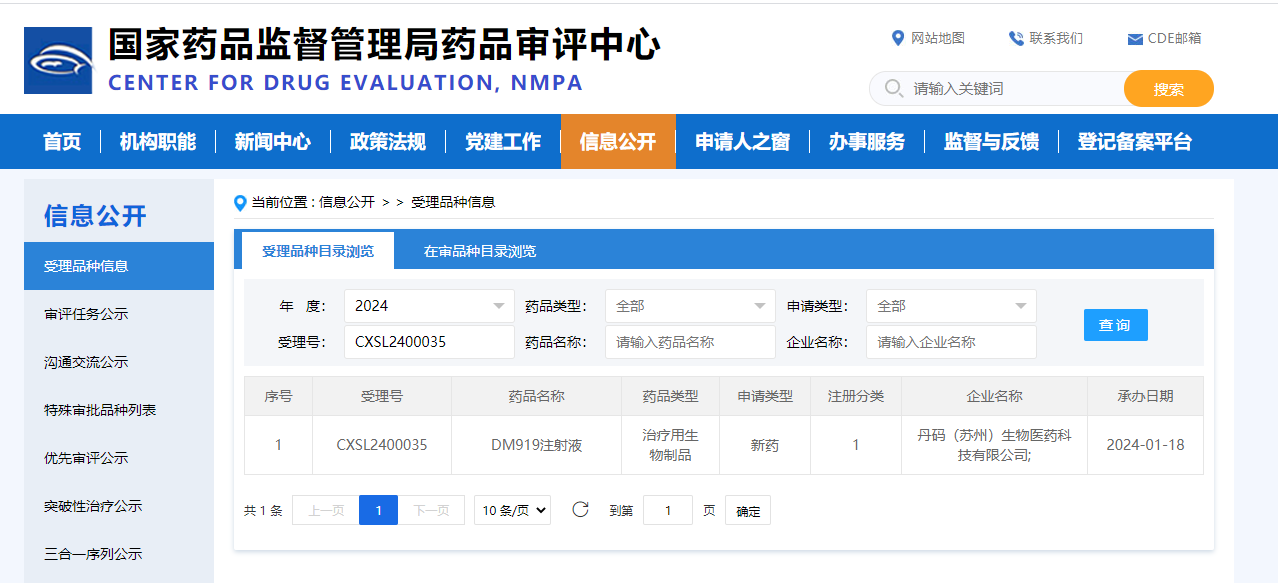
As the antibody CMC service provider for D2M Biotherapeutics, Zencore Biologics is honored to offer comprehensive CMC support from Sequence to IND submission, aiding D2M Biotherapeutics in obtaining IND approval for its new drug project.
Zencore Biologics sincerely wish that D2M Biotherapeutics will make smooth clinical progress and be launched as soon as possible, which will bring the hope of cure to patients. Zencore Biologics will always adhere to providing high-quality, efficient and comprehensive CDMO services for global partners.