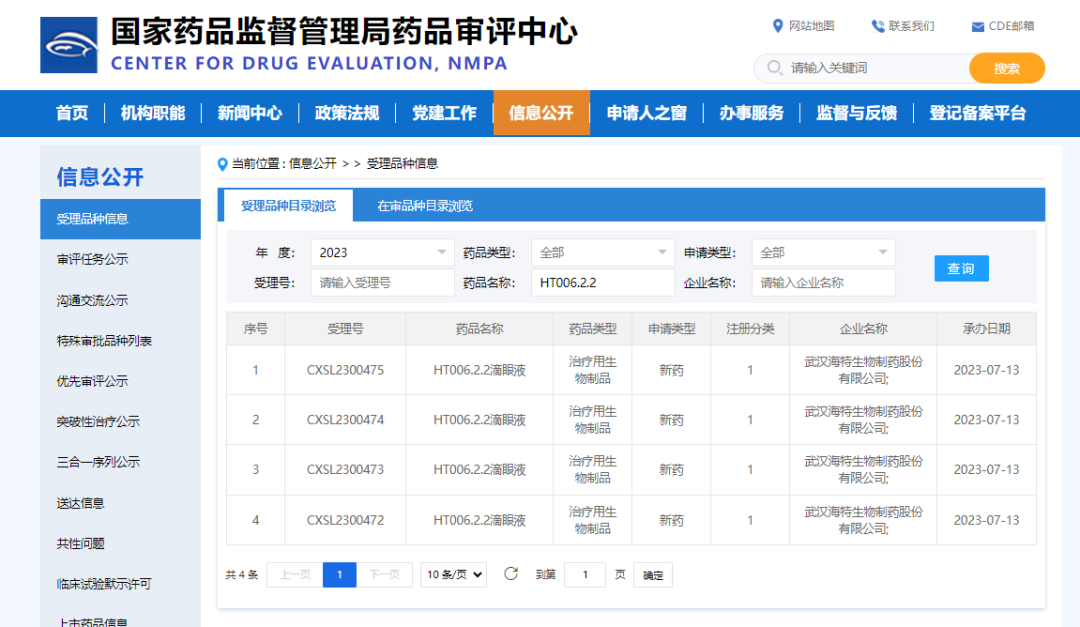
On October 09, 2023, in Wuhan, China, Wuhan HITECK Biopharmaceutical Co., Ltd. received approval from the National Medical Products Administration (NMPA) for the clinical trial application of a Class 1 therapeutic biologic new drug: HT006.2.2 Eye Drops (ZG023).
It is a great honor for Zencore Biologics to be the CMC service provider for HITECK biopharmaceutical, offering services in process and analytical method development, contributing to the successful IND approval of the HT006.2.2 (ZG023) new drug project.